 |
WARNINGTRIZIVIR contains 3 nucleoside analogs (abacavir sulfate, lamivudine, and zidovudine) and is intended only for patients whose regimen would otherwise include these 3 components. TRIZIVIR contains abacavir sulfate (ZIAGEN®), which has been associated with fatal hypersensitivity reactions (see WARNINGS ). Patients developing signs or symptoms of hypersensitivity (which include fever; skin rash; fatigue; gastrointestinal symptoms such as nausea, vomiting, diarrhea, or abdominal pain; and respiratory symptoms such as pharyngitis, dyspnea, or cough) should discontinue TRIZIVIR as soon as a hypersensitivity reaction is suspected. To avoid a delay in diagnosis and minimize the risk of a life-threatening hypersensitivity reaction, TRIZIVIR should be permanently discontinued if hypersensitivity cannot be ruled out, even when other diagnoses are possible (e.g., acute onset respiratory diseases, gastroenteritis, or reactions to other medications). Abacavir (as TRIZIVIR OR ZIAGEN) SHOULD NOT be restarted following a hypersensitivity reaction to abacavir because more severe symptoms will recur within hours and may include life-threatening hypotension and death. Severe or fatal hypersensitivity reactions can occur within hours after reintroduction of abacavir (as TRIZIVIR OR ZIAGEN) in patients who have no identified history or unrecognized symptoms of hypersensitivity to abacavir therapy (see WARNINGS , PRECAUTIONS : Information for Patients , and ADVERSE REACTIONS ). Zidovudine has been associated with hematologic toxicity including neutropenia and severe anemia, particularly in patients with advanced HIV disease (see WARNINGS ). Prolonged use of zidovudine has been associated with symptomatic myopathy. Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including abacavir, lamivudine, zidovudine, and other antiretrovirals (see WARNINGS ). TRIZIVIR alone or in combination with other antiretroviral agents is indicated for the treatment of HIV-1 infection. The indication for TRIZIVIR is based on analyses of surrogate markers in controlled studies with abacavir of up to 24 weeks in duration. At present, there are no results from controlled trials evaluating long-term suppression of HIV RNA or disease progression with abacavir. There are limited data on the use of this triple-combination regimen in patients with higher viral load levels (>100,000 copies/mL) at baseline. |
TRIZIVIR: TRIZIVIR Tablets contain the following 3 synthetic nucleoside analogues: abacavir sulfate (ZIAGEN), lamivudine (also known as EPIVIR® or 3TC), and zidovudine (also known as RETROVIR®, azidothymidine, or ZDV) with inhibitory activity against human immunodeficiency virus (HIV).
TRIZIVIR Tablets are for oral administration. Each film-coated tablet contains the active ingredients 300 mg of abacavir as abacavir sulfate, 150 mg of lamivudine, and 300 mg of zidovudine, and the inactive ingredients magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The tablets are coated with a film (Opadry® green 03B11434) that is made of FD&C Blue No. 2, hydroxypropyl methylcellulose, polyethylene glycol, titanium dioxide, and yellow iron oxide.
Abacavir Sulfate: The chemical name of abacavir sulfate is ( 1 S,cis) -4-[2-amino-6-(cyclopropylamino)-9 H -purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). Abacavir sulfate is the enantiomer with 1S, 4R absolute configuration on the cyclopentene ring. It has a molecular formula of (C 14 H 18 N 6 O) 2 ·H 2 SO 4 and a molecular weight of 670.76 daltons. It has the following structural formula:
 |
Abacavir sulfate is a white to off-white solid with a solubility of approximately 77 mg/mL in distilled water at 25°C.
In vivo, abacavir sulfate dissociates to its free base, abacavir. In this insert, all dosages for ZIAGEN (abacavir sulfate) are expressed in terms of abacavir.
Lamivudine: The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)2',3'-dideoxy, 3'-thiacytidine. It has a molecular formula of C 8 H 11 N 3 O 3 S and a molecular weight of 229.3 daltons. It has the following structural formula:
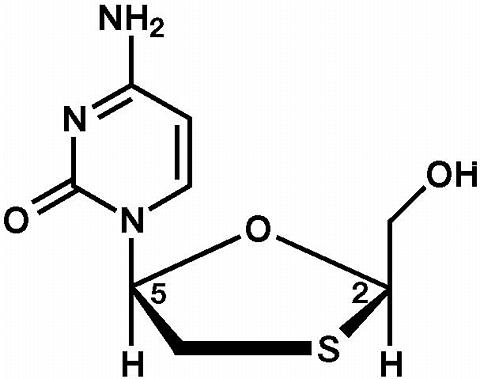 |
Lamivudine is a white to off-white crystalline solid with a solubility of approximately 70 mg/mL in water at 20°C.
Zidovudine: The chemical name of zidovudine is 3'-azido-3'-deoxythymidine. It has a molecular formula of C 10 H 13 N 5 O 4 and a molecular weight of 267.24 daltons. It has the following structural formula:
 |
Zidovudine is a white to beige, crystalline solid with a solubility of 20.1 mg/mL in water at 25°C.
Abacavir: Abacavir is a carbocyclic synthetic nucleoside analogue. Intracellularly, abacavir is converted by cellular enzymes to the active metabolite, carbovir triphosphate. Carbovir triphosphate is an analogue of deoxyguanosine-5'-triphosphate (dGTP). Carbovir triphosphate inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA. The lack of a 3'-OH group in the incorporated nucleoside analogue prevents the formation of the 5' to 3' phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated.
Lamivudine: Lamivudine is a synthetic nucleoside analogue. Intracellularly, lamivudine is phosphorylated to its active 5'-triphosphate metabolite, lamivudine triphosphate (L-TP). The principal mode of action of L-TP is inhibition of RT via DNA chain termination after incorporation of the nucleoside analogue. L-TP is a weak inhibitor of mammalian DNA polymerases-(alpha) and -(beta) and mitochondrial DNA polymerase-(gamma).
Zidovudine Zidovudine is a synthetic nucleoside analogue. Intracellularly, zidovudine is phosphorylated to its active 5'-triphosphate metabolite, zidovudine triphosphate (ZDV-TP). The principal mode of action of ZDV-TP is inhibition of RT via DNA chain termination after incorporation of the nucleoside analogue. ZDV-TP is a weak inhibitor of the mammalian DNA polymerase-(alpha) and mitochondrial DNA polymerase-(gamma) and has been reported to be incorporated into the DNA of cells in culture.
The relationship between in vitro susceptibility of HIV to abacavir, lamivudine, or zidovudine and the inhibition of HIV replication in humans has not been established.
Abacavir: The in vitro anti-HIV-1 activity of abacavir was evaluated against a T-cell tropic laboratory strain HIV-1 IIIB in lymphoblastic cell lines, a monocyte/macrophage tropic laboratory strain HIV-1 BaL in primary monocytes/macrophages, and clinical isolates in peripheral blood mononuclear cells. The concentration of drug necessary to inhibit viral replication by 50 percent (IC 50 ) ranged from 3.7 to 5.8 µM against HIV-1 IIIB, and was 0.26 ± 0.18 µM (1 µM = 0.28 mcg/mL) against 8 clinical isolates. The IC 50 of abacavir against HIV-1 BaL varied from 0.07 to 1.0 µM. Abacavir had synergistic activity in combination with amprenavir, nevirapine, and zidovudine, and additive activity in combination with didanosine, lamivudine, stavudine, and zalcitabine in vitro. Most of these drug combinations have not been adequately studied in humans.
Lamivudine: In vitro activity of lamivudine against HIV-1 was assessed in a number of cell lines (including monocytes and fresh human peripheral blood lymphocytes). IC 50 and IC 90 values (50% and 90% inhibitory concentrations) for lamivudine were 0.0006 mcg/mL to 0.034 mcg/mL and 0.015 to 0.321 mcg/mL, respectively. Lamivudine had anti-HIV-1 activity in all acute virus-cell infections tested.
In HIV-1-infected MT-4 cells, lamivudine in combination with zidovudine had synergistic antiretroviral activity.
Zidovudine: In vitro activity of zidovudine against HIV-1 was assessed in a number of cell lines (including monocytes and fresh human peripheral blood lymphocytes). The IC 50 and IC 90 values for zidovudine were 0.003 to 0.013 mcg/mL and 0.03 to 0.13 mcg/mL, respectively. Zidovudine had anti-HIV-1 activity in all acute virus-cell infections tested. However, zidovudine activity was substantially less in chronically infected cell lines. In cell culture drug combination studies, zidovudine demonstrates synergistic activity with, delavirdine, didanosine, indinavir, nelfinavir, nevirapine, ritonavir, saquinavir, and zalcitabine, and additive activity with interferon-alpha.
HIV-1 isolates with reduced sensitivity to abacavir, lamivudine, or zidovudine have been selected in vitro and were also obtained from patients treated with abacavir, lamivudine, zidovudine, or lamivudine plus zidovudine. The clinical relevance of genotypic and phenotypic changes associated with abacavir, lamivudine, or zidovudine therapy is currently under evaluation.
Abacavir: Genetic analysis of isolates from abacavir-treated patients showed point mutations in the reverse transcriptase gene that resulted in amino acid substitutions at positions K65R, L74V, Y115F, and M184V. Mutations M184V and L74V were most frequently observed in clinical isolates. Phenotypic analysis of HIV-1 isolates that harbored abacavir-associated mutations from 17 patients after 12 weeks of abacavir monotherapy exhibited a 3-fold decrease in susceptiblity to abacavir in vitro.
Lamivudine: Genotypic analysis of isolates selected in vitro and recovered from lamivudine-treated patients showed that the resistance was due to mutations in the HIV-1 reverse transcriptase gene at codon 184 from methionine to either isoleucine or valine.
Zidovudine: Genotypic analyses of the isolates selected in vitro and recovered from zidovudine-treated patients showed mutations, which result in 5 amino acid substitutions (M41L, D67N, K70R, K219Q, T215Y or F) in the HIV-1 reverse transcriptase gene. In general, higher levels of resistance were associated with greater number of mutations. In some patients harboring zidovudine-resistant virus at baseline, phenotypic sensitivity to zidovudine was restored by 12 weeks of treatment with lamivudine and zidovudine. Combination therapy with lamivudine plus zidovudine delayed the emergence of mutations conferring resistance to zidovudine.
Cross-resistance among certain reverse transcriptase inhibitors has been recognized.
Abacavir: Recombinant laboratory strains of HIV-1 (HXB2) containing multiple reverse transcriptase mutations conferring abacavir resistance exhibited cross-resistance to lamivudine, didanosine, and zalcitabine in vitro. For clinical information in treatment-experienced patients, see INDICATIONS AND USAGE : Description of Clinical Studies and PRECAUTIONS .
Lamivudine: Cross-resistance between lamivudine and zidovudine has not been reported. Cross-resistance to didanosine and zalcitabine has been observed in some patients harboring lamivudine resistant HIV-1 isolates. In some patients treated with zidovudine plus didanosine or zalcitabine, isolates resistant to multiple drugs, including lamivudine, have emerged (see under Zidovudine below).
Zidovudine HIV isolates with multidrug resistance to didanosine, lamivudine, stavudine, zalcitabine, and zidovudine were recovered from a small number of patients treated for >/=1 year with zidovudine plus didanosine or zidovudine plus zalcitabine. The pattern of genotypic resistant mutations with such combination therapies was different (A62V, V75I, F77L, F116Y, Q151M) from the pattern with zidovudine monotherapy, with the 151 mutation being most commonly associated with multidrug resistance. The mutation at codon 151 in combination with the mutations at 62, 75, 77, and 116 results in a virus with reduced susceptibility to, didanosine, lamivudine, stavudine, zalcitabine, and zidovudine.
TRIZIVIR In a single-dose, 3-way crossover bioavailability study of 1 TRIZIVIR tablet versus 1 ZIAGEN tablet (300 mg), 1 EPIVIR tablet (150 mg), plus 1 RETROVIR tablet (300 mg) administered simultaneously in healthy subjects (n = 24), there was no difference in the extent of absorption, as measured by the area under the plasma concentration-time curve (AUC) and maximal peak concentration (C max ), of all 3 components. One TRIZIVIR tablet was bioequivalent to 1 ZIAGEN tablet (300 mg), 1 EPIVIR tablet (150 mg), plus 1 RETROVIR tablet (300 mg) following single-dose administration to fasting healthy subjects (n = 24).
Abacavir Following oral administration, abacavir is rapidly absorbed and extensively distributed. Binding of abacavir to human plasma proteins is approximately 50%. Binding of abacavir to plasma proteins was independent of concentration. Total blood and plasma drug-related radioactivity concentrations are identical, demonstrating that abacavir readily distributes into erythrocytes. The primary routes of elimination of abacavir are metabolism by alcohol dehydrogenase to form the 5'-carboxylic acid and glucuronyl transferase to form the 5'-glucuronide.
Lamivudine Following oral administration, lamivudine is rapidly absorbed and extensively distributed. Binding to plasma protein is low. Approximately 70% of an intravenous dose of lamivudine is recovered as unchanged drug in the urine. Metabolism of lamivudine is a minor route of elimination. In humans, the only known metabolite is the trans-sulfoxide metabolite (approximately 5% of an oral dose after 12 hours).
Zidovudine Following oral administration, zidovudine is rapidly absorbed and extensively distributed. Binding to plasma protein is low. Zidovudine is eliminated primarily by hepatic metabolism. The major metabolite of zidovudine is 3'-azido-3'-deoxy-5'- O beta D -glucopyranuronosylthymidine (GZDV). GZDV area under the curve (AUC) is about 3-fold greater than the zidovudine AUC. Urinary recovery of zidovudine and GZDV accounts for 14% and 74% of the dose following oral administration, respectively. A second metabolite, 3'-amino-3'-deoxythymidine (AMT), has been identified in plasma. The AMT AUC was one fifth of the zidovudine AUC.
In humans, abacavir, lamivudine, and zidovudine are not significantly metabolized by cytochrome P450 enzymes.
The pharmacokinetic properties of abacavir, lamivudine, and zidovudine in fasting patients are summarized in Table 1.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TRIZIVIR may be administered with or without food. Administration with food in a single-dose bioavailability study resulted in lower C max , similar to results observed previously for the reference formulations. The average [90% Cl] decrease in abacavir, lamivudine and zidovudine C max was 32% [24% to 38%], 18% [10% to 25%], and 28% [13% to 40%], respectively, when administered with a high-fat meal, compared to administration under fasted conditions. Administration of TRIZIVIR with food did not alter the extent of abacavir, lamivudine, and zidovudine absorption (AUC), as compared to administration under fasted conditions (n =24).
TRIZIVIR: Because lamivudine and zidovudine require dose adjustment in the presence of renal insufficiency, TRIZIVIR is not recommended for use in patients with creatinine clearance </=50 mL/min (see PRECAUTIONS ).
See PRECAUTIONS : Pregnancy .
Zidovudine Zidovudine pharmacokinetics have been studied in a Phase 1 study of 8 women during the last trimester of pregnancy. As pregnancy progressed, there was no evidence of drug accumulation. The pharmacokinetics of zidovudine were similar to that of nonpregnant adults. Consistent with passive transmission of the drug across the placenta, zidovudine concentrations in neonatal plasma at birth were essentially equal to those in maternal plasma at delivery. Although data are limited, methadone maintenance therapy in 5 pregnant women did not appear to alter zidovudine pharmacokinetics. In a nonpregnant adult population, a potential for interaction has been identified (see CLINICAL PHARMACOLOGY : Drug Interactions ).
Abacavir and Lamivudine: No data are available on the pharmacokinetics of abacavir or lamivudine during pregnancy.
See PRECAUTIONS : Nursing Mothers .
Zidovudine: After administration of a single dose of 200 mg zidovudine to 13 HIV-infected women, the mean concentration of zidovudine was similar in human milk and serum.
Abacavir and Lamivudine: No data are available on the pharmacokinetics of abacavir or lamivudine in nursing mothers.
TRIZIVIR: TRIZIVIR is not intended for use in pediatric patients. TRIZIVIR should not be administered to adolescents who weigh less than 40 kg because it is a fixed-dose tablet that cannot be dose adjusted for this patient population (see PRECAUTIONS : Pediatric Use ).
The pharmacokinetics of abacavir, lamivudine, and zidovudine have not been studied in patients over 65 years of age.
Lamivudine and Zidovudine: A pharmacokinetic study in healthy male (n = 12) and female (n = 12) subjects showed no gender differences in zidovudine exposure (AUC(infinity)) or lamivudine AUC(infinity) normalized for body weight.
Abacavir: The pharmacokinetics of abacavir with respect to gender have not been determined.
Lamivudine: There are no significant racial differences in lamivudine pharmacokinetics.
Abacavir and Zidovudine: The pharmacokinetics of abacavir and zidovudine with respect to race have not been determined.
See PRECAUTIONS Drug Interactions .
The drug interactions described are based on studies conducted with the individual nucleoside analogues. In humans, abacavir, lamivudine, and zidovudine are not significantly metabolized by cytochrome P450 enzymes; therefore, it is unlikely that clinically significant drug interactions will occur with drugs metabolized through these pathways.
Abacavir: Due to their common metabolic pathways via glucuronyl transferase with zidovudine, 15 HIV-infected patients were enrolled in a crossover study evaluating single doses of abacavir (600 mg), lamivudine (150 mg), and zidovudine (300 mg) alone or in combination. Analysis showed no clinically relevant changes in the pharmacokinetics of abacavir with the addition of lamivudine or zidovudine or the combination of lamivudine and zidovudine. Lamivudine exposure (AUC decreased 15%) and zidovudine exposure (AUC increased 10%) did not show clinically relevant changes with concurrent abacavir.
Lamivudine and Zidovudine: No clinically significant alterations in lamivudine or zidovudine pharmacokinetics were observed in 12 asymptomatic HIV-infected adult patients given a single dose of zidovudine (200 mg) in combination with multiple doses of lamivudine (300 mg q 12 h).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TRIZIVIR is indicated alone or in combination with other antiretroviral agents for the treatment of HIV-1 infection. The indication for TRIZIVIR is based on analyses of surrogate markers in controlled studies with abacavir of up to 24 weeks in duration. At present, there are no results from controlled trials evaluating long-term suppression of HIV RNA or disease progression with therapy with abacavir. There are limited data on the use of this triple-combination regimen in patients with higher viral load levels (>100,000 copies/mL) at baseline (see Description of Clinical Studies for ZIAGEN).
There have been no clinical trials conducted with TRIZIVIR (see CLINICAL PHARMACOLOGY for information about bioequivalence of TRIZIVIR).
The following studies were conducted with the individual components of TRIZIVIR.
Therapy-Naive Adults CNAAB3003 was a multicenter, double-blind, placebo-controlled study in which 173 HIV-infected, therapy-naive adults were randomized to receive either ZIAGEN (300 mg twice daily), lamivudine (150 mg twice daily), and zidovudine (300 mg twice daily) or lamivudine (150 mg twice daily) and zidovudine (300 mg twice daily). The duration of double-blind treatment was 16 weeks. Study participants were: male (76%), Caucasian (54%), African-American (28%), and Hispanic (16%). The median age was 34 years, the median pretreatment CD4 cell count was 450 cells/mm 3 , and median plasma HIV-1 RNA was 4.5 log 10 copies/mL. Proportions of patients with plasma HIV-1 RNA <400 copies/mL (using Roche Amplicor HIV-1 MONITOR® Test) through 16 weeks of treatment are summarized in Figure 1.
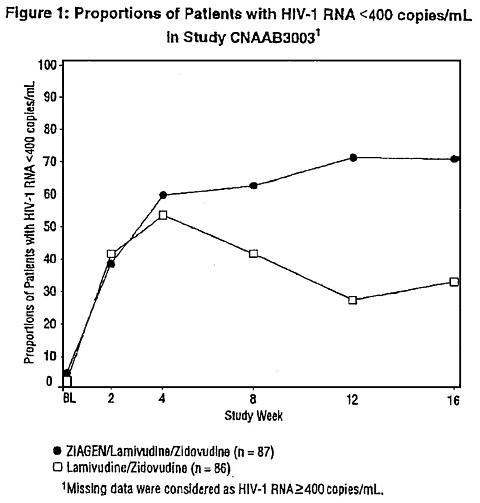 |
Preliminary findings from a second controlled study in therapy-naive adults were supportive of the efficacy of abacavir through 16 weeks of treatment.
Therapy-Experienced Pediatric Patients A randomized, double-blind study, CNAA3006, compared ZIAGEN plus lamivudine and zidovudine versus lamivudine and zidovudine in pediatric patients, most of whom were extensively pretreated with nucleoside analogue antiretroviral agents. Patients in this study had a limited response to abacavir.
Abacavir sulfate, one of the components of TRIZIVIR, has been associated with fatal hypersensitivity reactions. ABACAVIR (as TRIZIVIR or ZIAGEN) SHOULD NOT BE RESTARTED FOLLOWING A HYPERSENSITIVITY REACTION TO ABACAVIR (see WARNINGS , PRECAUTIONS , and ADVERSE REACTIONS ).
TRIZIVIR Tablets are contraindicated in patients with previously demonstrated hypersensitivity to any of the components of the product (see WARNINGS ).
Hypersensitivity Reaction: TRIZIVIR contains abacavir sulfate (ZIAGEN), which has been associated with fatal hypersensitivity reactions. Patients developing signs or symptoms of hypersensitivity (which include fever; skin rash; fatigue; gastrointestinal symptoms such as nausea, vomiting, diarrhea, or abdominal pain; and respiratory symptoms such as pharyngitis, dyspnea, or cough) should discontinue TRIZIVIR as soon as a hypersensitivity reaction is first suspected, and should seek medical evaluation immediately. To avoid a delay in diagnosis and minimize the risk of a life-threatening hypersensitivity reaction, TRIZIVIR should be permanently discontinued if hypersensitivity cannot be ruled out, even when other diagnoses are possible (e.g., acute onset respiratory diseases, gastroenteritis, or reactions to other medications). Abacavir (as TRIZIVIR or ZIAGEN) SHOULD NOT be restarted following a hypersensitivity reaction to abacavir because more severe symptoms will recur within hours and may include life-threatening hypotension and death.
Severe or fatal hypersensitivity reactions can occur within hours after reintroduction of abacavir (as TRIZIVIR or ZIAGEN) in patients who have no identified history or unrecognized symptoms of hypersensitivity to abacavir therapy.
When therapy with abacavir (as TRIZIVIR or ZIAGEN) has been discontinued for reasons other than symptoms of a hypersensitivity reaction, and if reinitiation of therapy is under consideration, the reason for discontinuation should be evaluated to ensure that the patient did not have symptoms of a hypersensitivity reaction. If hypersensitivity cannot be ruled out, abacavir (as TRIZIVIR or ZIAGEN) should NOT be reintroduced. If symptoms consistent with hypersensitivity are not identified, reintroduction can be undertaken with continued monitoring for symptoms of a hypersensitivity reaction. Patients should be made aware that a hypersensitivity reaction can occur with reintroduction of abacavir (as TRIZIVIR or ZIAGEN), and that reintroduction of abacavir (as TRIZIVIR or ZIAGEN) should be undertaken only if medical care can be readily accessed by the patient or others (see ADVERSE REACTIONS ).
In clinical trials, hypersensitivity reactions have been reported in approximately 5% of adult and pediatric patients receiving abacavir. Symptoms usually appear within the first 6 weeks of treatment with abacavir although these reactions may occur at any time during therapy (see PRECAUTIONS : Information for Patients and ADVERSE REACTIONS ).
Abacavir Hypersensitivity Reaction Registry To facilitate reporting of hypersensitivity reactions and collection of information on each case, an Abacavir Hypersensitivity Registry has been established. Physicians should register patients by calling 1-800-270-0425.
Lactic Acidosis/Severe Hepatomegaly with Steatosis Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including abacavir, lamivudine, zidovudine, and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering TRIZIVIR to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with TRIZIVIR should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Bone Marrow Suppression Since TRIZIVIR contains zidovudine, TRIZIVIR should be used with caution in patients who have bone marrow compromise evidenced by granulocyte count <1000 cells/mm 3 or hemoglobin <9.5 g/dL. Frequent blood counts are strongly recommended in patients with advanced HIV disease who are treated with TRIZIVIR. For HIV-infected individuals and patients with asymptomatic or early HIV disease, periodic blood counts are recommended.
Myopathy Myopathy and myositis, with pathological changes similar to that produced by HIV disease, have been associated with prolonged use of zidovudine, and therefore may occur with therapy with TRIZIVIR.
Other TRIZIVIR contains fixed doses of 3 nucleoside analogues: abacavir, lamivudine, and zidovudine and should not be administered concomitantly with abacavir, lamivudine, or zidovudine.
Because TRIZIVIR is a fixed-dose tablet, it should not be prescribed for adults or adolescents who weigh less than 40 kg or other patients requiring dosage adjustment.
The complete prescribing information for all agents being considered for use with TRIZIVIR should be consulted before combination therapy with TRIZIVIR is initiated.
Abacavir In clinical trials, patients with prolonged prior nucleoside reverse transcriptase inhibitor (NRTI) exposure or who had HIV-1 isolates that contained multiple mutations conferring resistance to NRTIs had limited response to abacavir. The potential for cross-resistance between abacavir and other NRTIs should be considered when choosing new therapeutic regimens in therapy-experienced patients (see MICROBIOLOGY : Cross-Resistance ).
Lamivudine In clinical trials and postmarketing experience, some patients with HIV infection who have chronic liver disease due to hepatitis B virus infection experienced clinical or laboratory evidence of recurrent hepatitis upon discontinuation of lamivudine. Consequences may be more severe in patients with decompensated liver disease.
TRIZIVIR Since TRIZIVIR is a fixed-dose tablet and the dosage of the individual components cannot be altered, patients with creatinine clearance </=50 mL/min should not receive TRIZIVIR.
Abacavir Patients should be advised that a Medication Guide and Warning Card summarizing the symptoms of abacavir hypersensitivity reactions should be dispensed by the pharmacist with each new prescription and refill of TRIZIVIR. The complete text of the Medication Guide is reprinted at the end of this document. Patients should be instructed to carry the Warning Card with them.
Patients should be advised of the possibility of a hypersensitivity reaction to abacavir (as TRIZIVIR or ZIAGEN) that may result in death. Patients developing signs or symptoms of hypersensitivity (which include fever; skin rash; fatigue; gastrointestinal symptoms such as nausea, vomiting, diarrhea, or abdominal pain; and respiratory symptoms such as sore throat, shortness of breath, or cough) should discontinue treatment with TRIZIVIR and seek medical evaluation immediately. Abacavir (as TRIZIVIR OR ZIAGEN) SHOULD NOT be restarted following a hypersensitivity reaction to abacavir because more severe symptoms will recur within hours and may include life-threatening hypotension and death. Patients who have interrupted abacavir (as TRIZIVIR or ZIAGEN) for reactions other than symptoms of hypersensitivity (for example, those who have an interruption in drug supply) should be made aware that a severe or fatal hypersensitivity reaction can occur with reintroduction of abacavir. Patients should be instructed not to reintroduce abacavir (as TRIZIVIR or ZIAGEN) without medical consultation and that reintroduction of abacavir (as TRIZIVIR or ZIAGEN) should be undertaken only if medical care can be readily accessed by the patient or others (see ADVERSE REACTIONS and WARNINGS ).
TRIZIVIR Patients should be informed that TRIZIVIR is not a cure for HIV infection and patients may continue to experience illnesses associated with HIV infection, including opportunistic infections. Patients should be advised that the use of TRIZIVIR has not been shown to reduce the risk of transmission of HIV to others through sexual contact or blood contamination.
Patients should be advised of the importance of taking TRIZIVIR as it is prescribed.
Zidovudine Patients should be informed that the important toxicities associated with zidovudine are neutropenia and/or anemia. They should be told of the extreme importance of having their blood counts followed closely while on therapy, especially for patients with advanced HIV disease.
TRIZIVIR No clinically significant changes to pharmacokinetic parameters were observed for abacavir, lamivudine, or zidovudine when administered together.
Abacavir Abacavir has no effect on the pharmacokinetic properties of ethanol. Ethanol decreases the elimination of abacavir causing an increase in overall exposure (see CLINICAL PHARMACOLOGY : Drug Interactions ).
Lamivudine TMP 160 mg/SMX 800 mg once daily has been shown to increase lamivudine exposure (AUC). The effect of higher doses of TMP/SMX on lamivudine pharmacokinetics has not been investigated (see CLINICAL PHARMACOLOGY ).
Zidovudine Coadministration of ganciclovir, interferon-alpha, and other bone marrow suppressive or cytotoxic agents may increase the hematologic toxicity of zidovudine (see CLINICAL PHARMACOLOGY ).
Abacavir Carcinogenicity studies in mice and rats are ongoing with abacavir.
Lamivudine Lamivudine long-term carcinogenicity studies in mice and rats showed no evidence of carcinogenic potential at exposures up to 10 times (mice) and 58 times (rats) those observed in humans at the recommended therapeutic dose.
Zidovudine Zidovudine was administered orally at 3 dosage levels to separate groups of mice and rats (60 females and 60 males in each group). Initial single daily doses were 30, 60, and 120 mg/kg per day in mice and 80, 220, and 600 mg/kg per day in rats. The doses in mice were reduced to 20, 30, and 40 mg/kg per day after day 90 because of treatment-related anemia, whereas in rats only the high dose was reduced to 450 mg/kg per day on day 91 and then to 300 mg/kg per day on day 279.
In mice, 7 late-appearing (after 19 months) vaginal neoplasms (5 nonmetastasizing squamous cell carcinomas, 1 squamous cell papilloma, and 1 squamous polyp) occurred in animals given the highest dose. One late-appearing squamous cell papilloma occurred in the vagina of a middle-dose animal. No vaginal tumors were found at the lowest dose.
In rats, 2 late-appearing (after 20 months), nonmetastasizing vaginal squamous cell carcinomas occurred in animals given the highest dose. No vaginal tumors occurred at the low or middle dose in rats. No other drug-related tumors were observed in either sex of either species.
At doses that produced tumors in mice and rats, the estimated drug exposure (as measured by AUC) was approximately 3 times (mouse) and 24 times (rat) the estimated human exposure at the recommended therapeutic dose of 100 mg every 4 hours.
Two transplacental carcinogenicity studies were conducted in mice. One study administered zidovudine at doses of 20 mg/kg per day or 40 mg/kg per day from gestation day 10 through parturition and lactation with dosing continuing in offspring for 24 months postnatally. At these doses, exposures were approximately 3 times the estimated human exposure at the recommended doses. After 24 months, at the 40-mg/kg-per-day dose, an increase in incidence of vaginal tumors was noted with no increase in tumors in the liver or lung or any other organ in either gender. These findings are consistent with results of the standard oral carcinogenicity study in mice, as described earlier. A second study administered zidovudine at maximum tolerated doses of 12.5 mg/day or 25 mg/day (~1000 mg/kg nonpregnant body weight or ~450 mg/kg of term body weight) to pregnant mice from days 12 through 18 of gestation. There was an increase in the number of tumors in the lung, liver, and female reproductive tracts in the offspring of mice receiving the higher dose level of zidovudine.
It is not known how predictive the results of rodent carcinogenicity studies may be for humans.
Abacavir Abacavir induced chromosomal aberrations both in the presence and absence of metabolic activation in an in vitro cytogenetic study in human lymphoctyes. Abacavir was mutagenic in the absence of metabolic activation, although it was not mutagenic in the presence of metabolic activation in a L5178Y/TK + /- mouse lymphoma assay. At systemic exposures approximately 9 times higher than that in humans at the therapeutic dose, abacavir was clastogenic in males and not clastogenic in females in an in vivo mouse bone marrow micronucleus assay. Abacavir was not mutagenic in bacterial mutagenicity assays in the presence and absence of metabolic activation.
Lamivudine Lamivudine was mutagenic in a L5178Y/TK + /- mouse lymphoma assay and clastogenic in a cytogenetic assay using cultured human lymphocytes. Lamivudine was negative in a microbial mutagenicity assay, in an in vitro cell transformation assay, in a rat micronucleus test, in a rat bone marrow cytogenetic assay, and in an assay for unscheduled DNA synthesis in rat liver.
Zidovudine Zidovudine was mutagenic in a L5178Y/TK + /- mouse lymphoma assay, positive in an in vitro cell transformation assay, clastogenic in a cytogenetic assay using cultured human lymphocytes, and positive in mouse and rat micronucleus tests after repeated doses. It was negative in a cytogenetic study in rats given a single dose.
Abacavir Abacavir administered to male and female rats had no adverse effects on fertility judged by conception rates at doses up to approximately 8-fold higher than that in humans at the therapeutic dose based on body surface area comparisons.
Lamivudine In a study of reproductive performance, lamivudine, administered to male and female rats at doses up to 130 times the usual adult dose based on body surface area considerations, revealed no evidence of impaired fertility judged by conception rates and no effect on the survival, growth, and development to weaning of the offspring.
Zidovudine Zidovudine, administered to male and female rats at doses up to 7 times the usual adult dose based on body surface area considerations, had no effect on fertility judged by conception rates.
Pregnancy Category C. There are no adequate and well-controlled studies of TRIZIVIR in pregnant women. Reproduction studies with abacavir, lamivudine, and zidovudine have been performed in animals (see Abacavir, Lamivudine, and Zidovudine sections below). TRIZIVIR should be used during pregnancy only if the potential benefits outweigh the risks.
Abacavir Studies in pregnant rats showed that abacavir is transferred to the fetus through the placenta. Developmental toxicity (depressed fetal body weight and reduced crown-rump length) and increased incidences of fetal anasarca and skeletal malformations were observed when rats were treated with abacavir at a dose 35 times higher than the human exposure, based on AUC (1000 mg/kg per day). In a fertility study, evidence of toxicity to the developing embryo and fetuses (increased resorptions, decreased fetal body weights) occurred only at 500 mg/kg per day. The offspring of female rats treated with abacavir at 500 mg/kg per day (beginning at embryo implantation and ending at weaning) showed increased incidence of stillbirth and lower body weights throughout life. In the rabbit, there was no evidence of drug-related developmental toxicity and no increases in fetal malformations at doses up to 8.5 times the human exposure, based on AUC.
Lamivudine Studies in pregnant rats and rabbits showed that lamivudine is transferred to the fetus through the placenta. Reproduction studies with orally administered lamivudine have been performed in rats and rabbits at 130 and 60 times, respectively, the usual adult dose (based on relative body surface area) and have revealed no evidence of teratogenicity. Some evidence of early embryolethality was seen in the rabbit at doses similar to those produced by the usual adult dose and higher, but there was no indication of this effect in the rat at orally administered doses up to 130 times the usual adult dose.
Zidovudine Reproduction studies with orally administered zidovudine in the rat and in the rabbit at doses up to 500 mg/kg per day revealed no evidence of teratogenicity with zidovudine. Zidovudine treatment resulted in embryo/fetal toxicity as evidenced by an increase in the incidence of fetal resorptions in rats given 150 or 450 mg/kg per day and rabbits given 500 mg/kg per day. The doses used in the teratology studies resulted in peak zidovudine plasma concentrations (after one-half of the daily dose) in rats 66 to 226 times, and in rabbits 12 to 87 times, mean steady-state peak human plasma concentrations (after one-sixth of the daily dose) achieved with the recommended daily dose (100 mg every 4 hours). In an additional teratology study in rats, a dose of 3000 mg/kg per day (very near the oral median lethal dose in rats of approximately 3700 mg/kg) caused marked maternal toxicity and an increase in the incidence of fetal malformations. This dose resulted in peak zidovudine plasma concentrations 350 times peak human plasma concentrations. No evidence of teratogenicity was seen in this experiment at doses of 600 mg/kg per day or less. Two rodent carcinogenicity studies were conducted (see Carcinogenesis, Mutagenesis, and Impairment of Fertility ).
Antiretroviral Pregnancy Registry To monitor maternal-fetal outcomes of pregnant women exposed to TRIZIVIR or other antiretroviral agents, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV infection.
Abacavir, Lamivudine, and Zidovudine Zidovudine is excreted in breast milk; abacavir is secreted into the milk of lactating rats; there are no data on lamivudine.
Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving TRIZIVIR.
TRIZIVIR is not intended for use in pediatric patients. TRIZIVIR should not be administered to adolescents who weigh less than 40 kg because it is a fixed-dose tablet that cannot be adjusted for this patient population.
Clinical studies of abacavir, lamivudine, and zidovudine did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. TRIZIVIR is not recommended for patients with impaired renal function (i.e., creatinine clearance </=50 mL/min; see PRECAUTIONS : Patients with Impaired Renal Function and DOSAGE AND ADMINISTRATION ).
Abacavir Hypersensitivity Reaction TRIZIVIR contains abacavir sulfate (ZIAGEN), which has been associated with fatal hypersensitivity reactions. Therapy with abacavir (as TRIZIVIR or ZIAGEN) SHOULD NOT be restarted following a hypersensitivity reaction because more severe symptoms will recur within hours and may include life-threatening hypotension and death. Patients developing signs or symptoms of hypersensitivity should discontinue treatment as soon as a hypersensitivity reaction is first suspected, and should seek medical evaluation immediately. To avoid a delay in diagnosis and minimize the risk of a life-threatening hypersensitivity reaction, TRIZIVIR should be permanently discontinued if hypersensitivity cannot be ruled out, even when other diagnoses are possible (e.g., acute onset respiratory diseases, gastroenteritis, or reactions to other medications).
Severe or fatal hypersensitivity reactions can occur within hours after reintroduction of abacavir (as TRIZIVIR or ZIAGEN) in patients who have no identified history or unrecognized symptoms of hypersensitivity to abacavir therapy (see WARNINGS and PRECAUTIONS : Information for Patients ).
When therapy with abacavir (as TRIZIVIR or ZIAGEN) has been discontinued for reasons other than symptoms of a hypersensitivity reaction, and if reinitiation of therapy is under consideration, the reason for discontinuation should be evaluated to ensure that the patient did not have symptoms of a hypersensitivity reaction. If hypersensitivity cannot be ruled out, abacavir (as TRIZIVIR or ZIAGEN) should NOT be reintroduced. If symptoms consistent with hypersensitivity are not identified, reintroduction can be undertaken with continued monitoring for symptoms of hypersensitivity reaction. Patients should be made aware that a hypersensitivity reaction can occur with reintroduction of abacavir (as TRIZIVIR or ZIAGEN), and that reintroduction of abacavir (as TRIZIVIR or ZIAGEN) should be undertaken only if medical care can be readily accessed by the patient or others (see WARNINGS ).
In clinical studies, approximately 5% of adult and pediatric patients receiving abacavir developed a hypersensitivity reaction. This reaction is characterized by the appearance of symptoms indicating multi-organ/body system involvement. Symptoms usually appear within the first 6 weeks of treatment with abacavir, although these reactions may occur at any time during therapy. Frequently observed signs and symptoms include fever; skin rash; fatigue; and gastrointestinal symptoms such as nausea, vomiting, diarrhea, or abdominal pain. Other signs and symptoms include malaise, lethargy, myalgia, myolysis, arthralgia, edema, cough, dyspnea, headache, and paresthesia. Some patients who experienced a hypersensitivity reaction were initially thought to have acute onset or worsening respiratory disease. The diagnosis of hypersensitivity reaction should be carefully considered for patients presenting with symptoms of acute onset respiratory diseases, even if alternative respiratory diagnoses (pneumonia, bronchitis, flu-like illness) are possible.
Physical findings include lymphadenopathy, mucous membrane lesions (conjunctivitis and mouth ulcerations), and rash. The rash usually appears maculopapular or urticarial but may be variable in appearance. Hypersensitivity reactions have occurred without rash.
Laboratory abnormalities include elevated liver function tests, increased creatine phosphokinase or creatinine, and lymphopenia. Anaphylaxis, liver failure, renal failure, hypotension, and death have occurred in association with hypersensitivity reactions. Symptoms worsen with continued therapy but often resolve upon discontinuation of abacavir.
Risk factors that may predict the occurrence or severity of hypersensitivity to abacavir have not been identified.
Selected clinical adverse events with a >/=5% frequency during therapy with ZIAGEN 300 mg twice daily and EPIVIR 150 mg twice daily and RETROVIR 300 mg twice daily compared with EPIVIR 150 mg twice daily and RETROVIR 300 mg twice daily from CNAAB3003 are listed in Table 3.
|
Laboratory Abnormalities Laboratory abnormalities (anemia, neutropenia, liver function test abnormalities, and CPK elevations) were observed with similar frequencies in the 2 treatment groups in studies CNAAB3003 and CNAAB3006. Mild elevations of blood glucose were more frequent in subjects receiving abacavir. In study CNAAB3003, triglyceride elevations (all grades) were more common on the abacavir arm (25%) than on the placebo arm (11%).
Other Adverse Events In addition to adverse events in Table 3 other adverse events observed in the expanded access program for abacavir were pancreatitis and increased GGT.
Lamivudine Plus Zidovudine In 4 randomized, controlled trials of lamivudine 300 mg per day plus zidovudine 600 mg per day, the following selected clinical and laboratory adverse events were observed (see Tables 4 and 5).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pancreatitis was observed in 3 of the 656 adult patients (<0.5%) who received lamivudine in controlled clinical trials.
Selected laboratory abnormalities observed during therapy are listed in Table 5.
|
||||||||||||||||||||||||
Lamivudine and Zidovudine The following events have been identified during post-approval use of lamivudine and/or zidovudine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to lamivudine and/or zidovudine.
Endocrine and Metabolic Hyperglycemia.
General Sensitization reactions (including anaphylaxis), vasculitis.
Hepatobiliary Tract and Pancreas Lactic acidosis and hepatic steatosis (see WARNINGS ), pancreatitis.
Musculoskeletal Muscle weakness, CPK elevation, rhabdomyolysis.
Nervous Seizures.
Skin Alopecia, erythema multiforme, Stevens-Johnson syndrome, urticaria.
Abacavir There is no known antidote for abacavir. It is not known whether abacavir can be removed by peritoneal dialysis or hemodialysis.
Lamivudine One case of an adult ingesting 6 grams of lamivudine was reported; there were no clinical signs or symptoms noted and hematologic tests remained normal. It is not known whether lamivudine can be removed by peritoneal dialysis or hemodialysis.
Zidovudine Acute overdoses of zidovudine have been reported in pediatric patients and adults. These involved exposures up to 50 grams. The only consistent findings were nausea and vomiting. Other reported occurrences included headache, dizziness, drowsiness, lethargy, and confusion. Hematologic changes were transient. All patients recovered. Hemodialysis and peritoneal dialysis appear to have a negligible effect on the removal of zidovudine while elimination of its primary metabolite, GZDV, is enhanced.
A Medication Guide and Warning Card that provide information about recognition of hypersensitivity reactions should be dispensed with each new prescription and refill. To facilitate reporting of hypersensitivity reactions and collection of information on each case, an Abacavir Hypersensitivity Registry has been established. Physicians should register patients by calling 1-800-270-0425.
The recommended oral dose of TRIZIVIR for adults and adolescents is 1 tablet twice daily. TRIZIVIR is not recommended in adults or adolescents who weigh less than 40 kg because it is a fixed-dose tablet.
Dose Adjustment Because it is a fixed-dose tablet, TRIZIVIR should not be prescribed for patients requiring dosage adjustment such as those with creatinine clearance </=50 mL/min or those experiencing dose-limiting adverse events.
TRIZIVIR is available as tablets. Each tablet contains 300 mg of abacavir as abacavir sulfate, 150 mg of lamivudine, and 300 mg of zidovudine. The tablets are blue-green capsule-shaped, film-coated, and imprinted with GX LL1 on one side with no markings on the reverse side. They are packaged as follows:
Bottles of 60 Tablets (NDC 0173-0691-00)
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature).
Glaxo Wellcome Inc., Research Triangle Park, NC 27709
US Patent Nos. 5,047,407; 5,905,082; 4,724,232; 4,818,538; 4,833,130; 4,837,208; 5,034,394; and 5,089,500
Lamivudine is manufactured under agreement fromBioChem Pharma Inc.
Laval, Quebec, Canada
©Copyright 2000, Glaxo Wellcome Inc. All rights reserved.
November 2000/RL-878
Generic name: abacavir sulfate, lamivudine, and zidovudine
Read the Medication Guide you get each time you fill your prescription for Trizivir. There may be new information since you filled your last prescription.
Trizivir contains abacavir, which is also called Ziagen®. About 1 in 20 patients (5%) who take abacavir (as Trizivir or Ziagen) will have a serious allergic reaction (hypersensitivity reaction) that may cause death if the drug is not stopped right away.
You may be having this reaction if:
If you think you may be having a reaction, STOP taking Trizivir and call your doctor right away.
If you stop treatment with Trizivir because of this serious reaction, NEVER take abacavir (as Trizivir or Ziagen) again. If you take any of these medicines again after you have had this serious reaction, you could die within hours.
Some patients who have stopped taking abacavir (as Trizivir or Ziagen) and who have then started taking abacavir again have had serious or life-threatening allergic (hypersensitivity) reactions. If you must stop treatment with Trizivir for reasons other than symptoms of hypersensitivity, do not begin taking it again without talking to your health care provider. If your health care provider decides that you may begin taking abacavir (as Trizivir or Ziagen) again, you should do so only in a setting with other people to get access to a doctor, if needed.
A written list of these symptoms is on the Warning Card your pharmacist gives you. Carry this Warning Card with you.
Trizivir can have other serious side effects. Be sure to read the section below entitled "What are the possible side effects of Trizivir?"
Trizivir is a medicine used to treat HIV infection. Trizivir includes 3 medicines: Ziagen (abacavir), Epivir® (lamivudine or 3TC), and Retrovir® (zidovudine, AZT, or ZDV).
All 3 of these medicines are called nucleoside analogue reverse transcriptase inhibitors (NRTIs). When used together, they help lower the amount of HIV in your blood. This helps to keep your immune system as healthy as possible so it can fight infection.
Different combinations of medicines are used to treat HIV infection. You and your doctor should discuss which combination of medicines is best for you.
Trizivir does not cure HIV infection or AIDS. Trizivir has not been studied long enough to know if it will help you live longer or have fewer of the medical problems that are associated with HIV infection or AIDS. Therefore, you must see your health care provider regularly.
Do not take Trizivir if you have ever had a serious allergic reaction (a hypersensitivity reaction) to any of the medicines that make up Trizivir, especially Ziagen (abacavir). If you have had such a reaction, return all of your unused Trizivir to your doctor or pharmacist.
Do not take Trizivir if you weigh less than 90 pounds.
To help make sure that your anti-HIV therapy is as effective as possible, take your Trizivir exactly as your doctor prescribes it. Do not skip any doses.
The usual dosage is 1 tablet twice a day. You can take Trizivir with food or on an empty stomach.
If you miss a dose of Trizivir, take the missed dose right away. Then, take the next dose at the usual scheduled time. Do not let your Trizivir run out. The amount of virus in your blood may increase if your anti-HIV drugs are stopped, even for a short time. Also, the virus in your body may become harder to treat.
Do not take Epivir, Retrovir, Combivir®, or Ziagen while taking Trizivir. These medicines are already in Trizivir.
Practice safe sex while using Trizivir. Do not use or share dirty needles. Trizivir does not reduce the risk of passing HIV to others through sexual contact or blood contamination.
Talk to your doctor if you are pregnant or if you become pregnant while taking Trizivir. Trizivir has not been studied in pregnant women. It is not known whether Trizivir will harm the unborn child.
Mothers with HIV should not breastfeed their babies because HIV is passed to the baby in breast milk. Also, Trizivir can be passed to babies in breast milk and could cause the child to have side effects.
Life-threatening allergic reaction. Trizivir contains abacavir, which is also called Ziagen. Abacavir has caused some people to have a life-threatening allergic reaction (hypersensitivity reaction) that can cause death. How to recognize a possible reaction and what to do are discussed in "What is the most important information I should know about Trizivir?" at the beginning of this Medication Guide.
Lactic acidosis and severe liver problems. The medicines in Trizivir can cause a serious condition called lactic acidosis and, in some cases, this condition can cause death. Nausea and tiredness that don't get better may be symptoms of lactic acidosis. Women are more likely than men to get this serious side effect.
Blood problems. Retrovir, one of the medicines in Trizivir, can cause serious blood cell problems. These include reduced numbers of white blood cells (neutropenia) and extremely reduced numbers of red blood cells (anemia). These blood cell problems are especially likely to happen in patients with advanced HIV disease or AIDS.
Your doctor should be checking your blood cell counts regularly while you are taking Trizivir. This is especially important if you have advanced HIV or AIDS. This is to make sure that any blood cell problems are found quickly.
Muscle weakness. Retrovir, one of the medicines in Trizivir, can cause muscle weakness. This can be a serious problem.
Other side effects. Trizivir can cause other side effects. The most common side effects of taking the medicines in Trizivir together are nausea, vomiting, diarrhea, loss of appetite, weakness or tiredness, headache, dizziness, pain or tingling of the hands or feet, and muscle and joint pain.
This listing of side effects is not complete. Your doctor or pharmacist can discuss with you a more complete list of side effects with Trizivir.
Ask a health care professional about any concerns about Trizivir. If you want more information, ask your doctor or pharmacist for the labeling for Trizivir that was written for health care professionals.
Do not use Trizivir for a condition for which it was not prescribed. Do not give Trizivir to other persons.
Glaxo Wellcome Inc. Research Triangle Park, NC 22709
This Medication Guide has been approved by the US Food and Drug Administration.